
The setting was a single-center pediatric referral facility, and admissions occurred between May 1 and July 15, 2021.
#MESSENGER RNA SERIES#
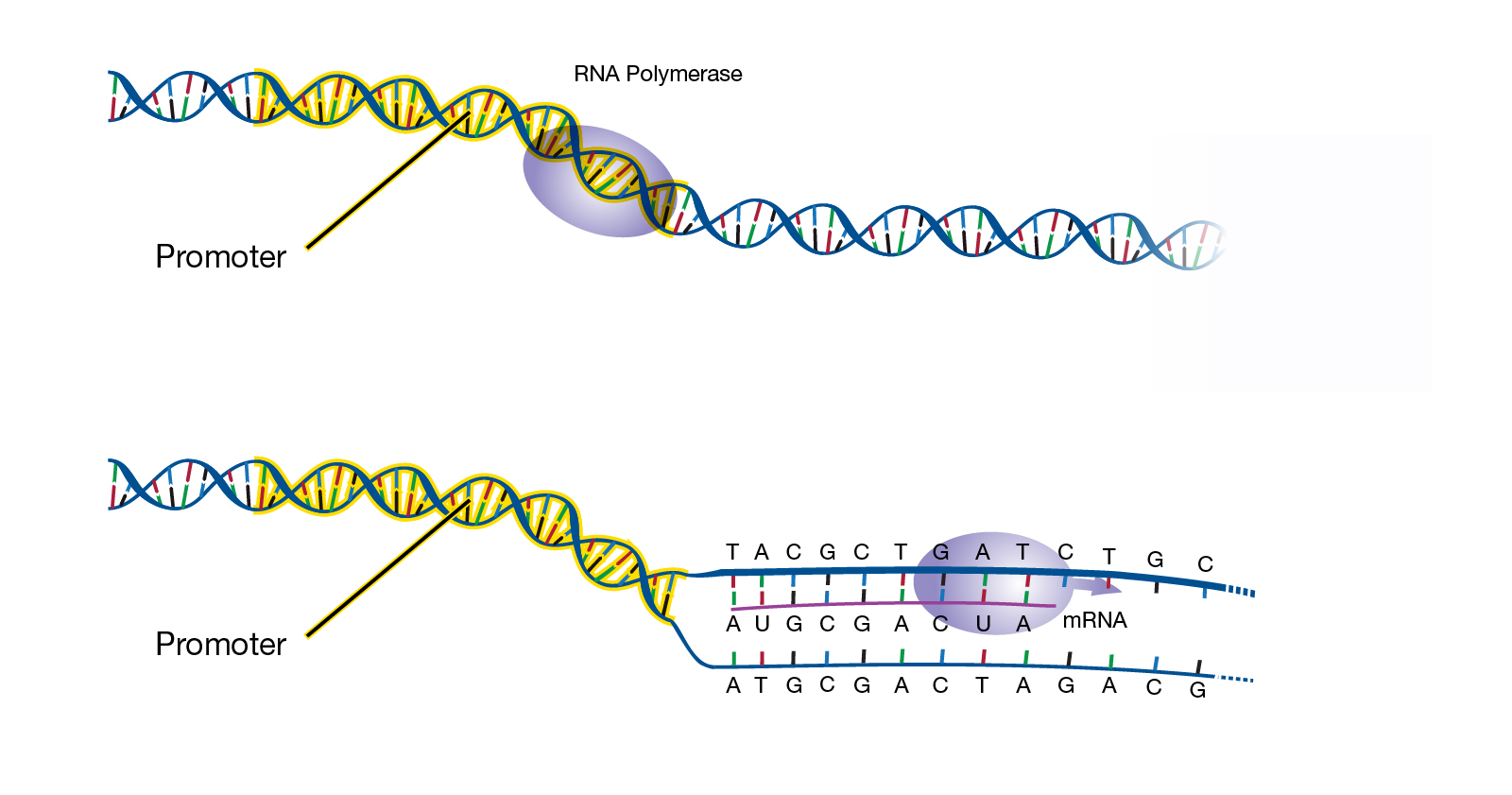
Objective To review results of comprehensive cardiac imaging in children with myocarditis after COVID-19 vaccine.ĭesign, Setting, and Participants This study was a case series of children younger than 19 years hospitalized with myocarditis within 30 days of BNT162b2 messenger RNA COVID-19 vaccine. Initial reports showed that the vaccine was well tolerated without serious adverse events however, cases of myocarditis have been reported since approval. Importance The BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine was authorized on May 10, 2021, for emergency use in children aged 12 years and older. Shared Decision Making and Communication.Scientific Discovery and the Future of Medicine.Health Care Economics, Insurance, Payment.Clinical Implications of Basic Neuroscience.Challenges in Clinical Electrocardiography.This article surveys the design, mode of action, and capabilities of state-of-the-art mRNA vaccines, focusing on the paradigm of influenza prophylaxis. Messenger RNA vaccines induce balanced immune responses including B cells, helper T cells, and cytotoxic T lymphocytes, rendering them an extremely adaptable platform. The formation of native antigen in situ affords great versatility, including intracellular localization, membrane association, posttranslational modification, supra-molecular assembly, or targeted structural optimization of delivered antigen. (3) RNA can be tailored to provide potent adjuvant stimuli to the innate immune system by direct activation of RNA-specific receptors this may reduce the need for additional adjuvants. This greatly reduces general complications associated with biological vaccine production, such as handling of infectious agents, genetic variability, environmental risks, or restrictions to vaccine distribution.

(2) mRNA vaccines are synthetically produced by an enzymatic process, just requiring information about the nucleic acid sequence of the desired antigen.
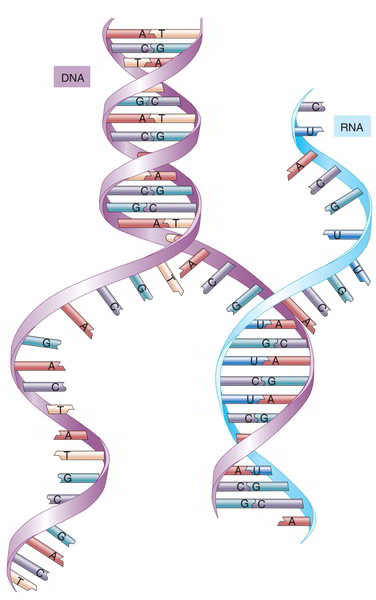
These characteristics also guarantee tight control over their immunogenic profile (including avoidance of vector-specific immune responses that could interfere with repeated administration), pharmacokinetics, and dosing. In the environment and upon physical contact, RNA is rapidly degraded by ubiquitous RNases and cannot persist. At the conceptual level, mRNA-based vaccines-more than other genetic vectors-combine the simplicity, safety, and focused immunogenicity of subunit vaccines with favorable immunological properties of live viral vaccines: (1) mRNA vaccines are molecularly defined and carry no excess information. These data indicate wider applicability to infectious disease and should encourage continued translation of mRNA-based prophylactic vaccines into human clinical trials. Twenty years after the demonstration that messenger RNA (mRNA) was expressed and immunogenic upon direct injection in mice, the first successful proof-of-concept of specific protection against viral infection in small and large animals was reported.


 0 kommentar(er)
0 kommentar(er)
